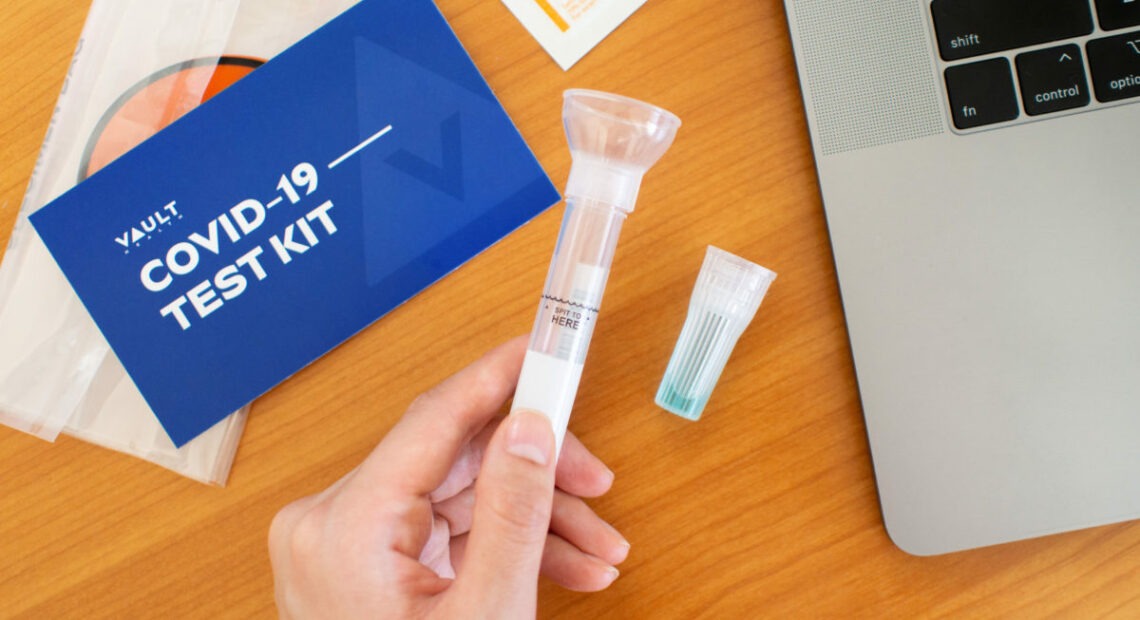
At-Home COVID Testing Is Growing. Can It Help Turn The Tide On The Pandemic?
BY FEDOR KOSSAKOVSKI / PBS NewsHour
If you’ve ever waited in a long line to receive a test for the coronavirus, or tried to get one and couldn’t, or waited a week to get the results, you may have wondered why it’s not easier and more convenient. In recent weeks, the Food and Drug Administration began approving over-the-counter COVID-19 tests for Americans to use at home, part of a wave of new options that could play a role in catching infections that might otherwise go undetected.
When President Donald Trump said back in March that “anybody that wants a test can get a test,” during a visit to the Centers for Disease Control and Prevention, it was not true. And depending on where you live, it’s still not true. Since the spring, the availability of coronavirus testing has improved dramatically overall, but experts say the country is still not doing enough testing to contain the virus’ rampant spread. And getting a test at a drive-thru site or doctor’s office usually requires access to transportation and time to wait — things that may be more difficult to come by for lower-income or more vulnerable populations — and also comes with the risk of potentially exposing health care workers to the virus, too.
The coronavirus tests most of us have heard about are based on the polymerase chain reaction, PCR, a chemical amplification technique that can detect even trace amounts of genetic material with very high specificity and sensitivity. It is the gold standard of diagnostic tests for infectious diseases, but it takes time to collect a sample, prepare it and run it for hours on sophisticated laboratory equipment.
The Trump administration has repeatedly come under fire for its lack of a national testing strategy, having left a cornerstone of pandemic mitigation largely up to states to set up their rules, infrastructure and process. “There’s been no organization centrally to help make sure that the right supplies are in the right place at the right time,” said Jason Feldman, founder and CEO of Vault Health. Now a year since the virus emerged in China, and days away from a U.S. presidential transition, cases are sky-high and testing data remains somewhat patchy.
At-home testing aims to solve some of these hurdles, but not all tests are equal nor are they all useful for the same situations.
So, what are the options for at-home tests out there now, and how do they differ from the standard PCR tests?
Telehealth company Vault Health has partnered with health departments in states like Minnesota to deploy at-home tests for the coronavirus to residents. Courtesy Hawaiian Airlines/Vault Health
Collected at home, tested in a lab
In April, Minnesota Gov. Tim Walz announced a “moonshot” of testing 20,000 Minnesotans a day for COVID, and in late June, the state’s Department of Health started hitting that goal with the help of the Mayo Clinic and the University of Minnesota. But with the state’s caseload plateauing amid looming predictions of a winter surge, Daniel Huff, assistant commissioner of the Health Protection Bureau, soon realized they would need to get the number closer to 40,000 per day.
One of the main pinch points in the process identified by Huff and his team was not their capacity for running samples on the PCR machines, but how you collect and prepare the samples for processing, Huff said.
So, in the summer, they decided to try an alternative to the ubiquitous nasal swabs: At-home collection of saliva for PCR testing, partnering with telehealth company Vault Health, who would provide the professional supervision required by the FDA for these kinds of tests.
“The mouth is a loaded cesspool of virus if you’re sick,” Feldman said.
The key difference is that a patient can easily self-administer the sample collection by spitting into a tube at home and mailing the kit back to a lab for processing, eliminating the need for a PPE-clad health care worker to get up close and personal to a possibly infected person’s face.
The test is highly sensitive and specific, performing as well as PCR tests on nasal swabs. Vault Health’s process is based on Rutgers University’s RUCDR Infinite Biologics test, the first saliva-based PCR test that received an Emergency Use Authorization from the Food and Drug Administration, though there are now other similar tests with that approval as well.
When someone signs up online for a test, a kit is shipped to the patient, who then teleconferences with a health care professional to ensure the sample is collected correctly. After the patient mails the kit back to the lab, Vault Health tries to deliver results within 48 hours of its arrival at the lab.
Vault Health has processed about 800,000 tests for Minnesotans as of early January 2021, the company says. It has similar programs partnered with state departments of health with New Mexico and Wisconsin. West Virginia also tried partnering with Vault, but last month decided to stop the program due to what the state health secretary said was a sometimes five-day lag, and search for quicker, more accessible options.
Other states, like New Jersey, have worked with private companies to provide at-home testing for their residents. Partnering with Vault Health, Minnesota also built a laboratory in the state to process the saliva samples locally.
After launching a few smaller trials in the area, the Minnesota Department of Health in November opened up at-home kits to anyone living in the state.
“We overwhelmed UPS,” Huff said, noting the state sent out 150,000 test kits by mail in the first week. “None of us anticipated the demand that we would see.”
Vault Health is widening the scope of its work after testing the method in professional sports leagues such as the PGA, MLS and MLB. Other companies, like e7 Health and Vitagene, also now offer at-home spit collection, and companies like EverlyWell are doing at-home nasal swab collection.
Processing results at home
Though Vault Health’s approach to COVID-19 testing minimizes contact for potentially infected people, it still takes time to transport a sample collected at home to a centralized lab. Up until recently, that was the only way the FDA had authorized “at-home” testing.
In November, the FDA gave its first EUA to a truly at-home COVID test, which allows the patient to collect the sample, process it and get results.
Made by biotech company Lucira Health, this test requires a patient swab their own nostrils, stir the sample into a vial, and plug it into a small box that performs the necessary chemistry. After about 30 minutes, lights indicate a positive, negative or invalid result.
Although it also detects viral RNA, this test uses a different chemistry than the usual PCR process, called loop-mediated isothermal amplification (LAMP). This difference means simpler equipment can be used and without pre-processing the sample like technicians would in a lab in order to run a PCR test. But this skipped step also means the Lucira Health test is a bit less sensitive than the gold-standard PCR tests – meaning it can give more false negatives.
“Purification of the nucleic acid is one of the steps that helps increase the sensitivity of the final reaction, the PCR. So, whenever you skip that step, you get a faster test, but you’re going to suffer a little bit on the sensitivity,” said Gary Procop, medical director of clinical virology at the Cleveland Clinic, who helped independently evaluate the Lucira Health test for its FDA authorization.
“But that was predicted from the start,” Procop added.
No diagnostic test is perfect, and given the chance of a false negative, it’s important for a patient to have that context when getting their results. Companies and health care providers should “put guardrails around that negative test and tell somebody, ‘Hey, if you’ve got signs and symptoms, even if this test is negative you should stay isolated for 14 days,’” Procop said.
Procop thought the Lucira Health test would be useful in similar ways as at-home saliva collection: for conserving PPE for health care workers and minimizing others’ exposure to potentially infected patients. The company is scaling up production now and hopes to make the test available, but only with a prescription, in the second quarter of 2021.
Possibilities for antigen tests
Besides at-home tests that look for viral RNA, there are also antigen tests, which look for the presence of coronavirus proteins in a sample. These products look like strips of paper onto which you apply a nasal swab or saliva sample. A few minutes later, lines develop on the strip if the protein is present.
The technique is called a lateral flow assay and is famously used in at-home pregnancy tests – no fancy sample prep or machinery required. The tradeoff for this simplicity is that these antigen tests have lower sensitivity than the nucleic acid tests, meaning there are more false negatives (there can also be some false positives, though generally antigen tests are as good at discerning the coronavirus from something else as the gold-standard PCR).
Despite the lower sensitivity, these cheaper, easier-to-manufacture tests could be used for wide-scale screening of the general public instead of diagnosing individual cases, said Amesh Adalja, an infectious disease expert at the Johns Hopkins Center for Health Security.
“Most asymptomatic people are not getting tested today. I didn’t get tested today,” Adalja said. “This would be something that you do on a day-to-day basis: You brush your teeth, test yourself, and then you go out the door.” A negative antigen test wouldn’t necessarily mean you don’t have the virus, but should catch those with the highest viral loads.
Adalja estimates many millions of these antigen tests could be done daily nationwide and would supplement diagnostic testing for symptomatic people, which is the main approach to testing currently in the U.S. At-home antigen testing could help curb the transmission of SARS-CoV-2 more effectively from asymptomatic “silent spreaders,” as long as the people who get positive tests take the appropriate actions.
Some health experts worry that this kind of testing without a prescription would mean some people won’t report their infection status to public health departments and we’d lose track of cases, but Adalja thinks that’s not the most important point. By giving people the ability to test frequently at home, Adalja said, coronavirus antigen tests would empower the public to make the right decision and isolate after a positive test.
This scenario may be on the horizon, as the FDA last month approved its second fully at-home coronavirus test: the Ellume rapid antigen test, which does not require a prescription and will be available over-the-counter soon. This, Adalja said, is a step in the right direction, though the $30 price tag is still too high to be used for regular screening.
The cost “makes it something that not everyone can use on a daily basis,” Adalja said. “We want something in the $5 or less range.” A cheaper at-home antigen test – the Abbott BinaxNOW – was approved one day after the Ellume product, but it requires a prescription.
Broadening access
Whatever the flavor of your at-home test, one of the main benefits is offering better options to underserved communities. For example, someone working an essential job bagging groceries and then taking care of their kids at home may not have time to go to wait in long lines at a drive-thru testing site.
Huff said that equity is “a cornerstone” of Minnesota’s COVID response: “We look at who are we testing, who are we not testing, how do we do a better job of reaching those people that are not getting tested right now? Are there barriers that we haven’t even thought of that we can remove?”
A big potential barrier for many is the cost of these at-home tests. Though the CARES Act sets up reimbursement for the cost of testing for both insured and uninsured individuals, that hasn’t always worked out in practice. Medical providers may charge patients for other tests to rule out other pathogens that are not covered.
“There’s been a lot of confusion around that. It has not always been applied accurately,” Huff said. For Minnesota’s at-home saliva collection and testing program, the state hammered out a contract with Vault Health directly.
“No Minnesotan will receive a bill for these tests, regardless of your insurance status,” Huff said.
Feldman said much of the cost of the Vault Health test, retailing at $119 if ordered online by an individual, comes from overnight shipping and the telehealth supervision.
“When we work with states and companies, we can get it down into the $70 range,” Feldman said. The company is exploring other ways to lower the price, like pooling samples from areas with lower positivity rates to conserve resources.
The Lucira Health at-home test will retail at $50, which Procop thinks would still be a chal
lenge for some. “That’s somebody’s grocery bill for a few days, if not longer if you’re extremely underserved,” he said.
Still, Procop thinks the Lucira Health test is a good expansion for at-home testing options, provided the company can deliver.
“It’s all a question of their ability to scale up,” Procop said. “They got a goose that laid a golden egg and now everybody wants a golden egg.”
Adalja doesn’t think these at-home diagnostic tests – whether just collection or fully at-home – are really going to change the game much on the public health scale. For that, he said, you would need those cheap antigen tests to be widely available around the country.
“This would democratize testing,” Adalja said. “I would like to see the president-elect issue an executive order that we want the FDA to do everything in their power to allow rapid home tests to be used by asymptomatic individuals in their homes.”
The Biden-Harris transition team’s coronavirus response plan emphasizes the need to make sure “all Americans have access to regular, reliable, and free testing,” including “at home tests and instant tests, so we can scale up our testing capacity by orders of magnitude.”
Overall, Procop emphasized, “a national testing strategy would address how we get testing in underserved populations.”
Fedor Kossakovski is a producer with Miles O’Brien Productions and a freelance science writer.
Copyright 2021 PBS NewsHour. To see more, visit pbs.org/newshour















